1964年Sweet 首先報告一種疾病, 特點為發燒, 白血球增高, 疼痛性紅色丘疹, 病理切片可見嗜中性球浸潤的現象。故名 Sweet’s syndrome。
史維特症候群 Sweet’s syndrome臨床上可見急性疼痛性紅色丘疹在頭頸, 手臂及下肢, 軀幹較少受侵犯, 約一半的病人有發燒及關節酸痛, 有部份病人會合併虹彩炎, 結膜炎等症狀。
INTRODUCTION — Sweet syndrome (acute febrile neutrophilic dermatosis) is an uncommon inflammatory disorder characterized by the abrupt appearance of painful, edematous, and erythematous papules, plaques, or nodules on the skin. Fever and leukocytosis frequently accompany the cutaneous lesions. In addition, involvement of the eyes, musculoskeletal system, and internal organs may occur.
The epidemiology, clinical features, and diagnosis of Sweet syndrome will be discussed here. Information on the treatment and prognosis of Sweet syndrome and an overview of other neutrophilic dermatoses are available separately. (See "Neutrophilic dermatoses".)
CLASSIFICATION — Sweet syndrome was first described by Dr. Robert Douglas Sweet in 1964, who documented the development of an acute inflammatory skin eruption with fever and leukocytosis in eight women, several of whom had preceding upper respiratory or gastrointestinal infections [1]. Since then, Sweet syndrome has been observed in association with a broad range of disorders. As a result, some authors divide Sweet syndrome into three subtypes based upon etiology:
- Classical Sweet syndrome
- Malignancy-associated Sweet syndrome
- Drug-induced Sweet syndrome
Classical Sweet syndrome — Classical Sweet syndrome (also referred to as idiopathic Sweet syndrome) constitutes the majority of cases of Sweet syndrome and is defined as Sweet syndrome that meets the established diagnostic criteria and is not associated with malignancy or drug exposure [2]. Classical Sweet syndrome may occur in the setting of a variety of medical conditions. The conditions that are most frequently associated with classical Sweet syndrome are listed below [2-5]:
- Infections (particularly upper respiratory tract and gastrointestinal infections; Sweet syndrome usually develops one to three weeks after infection [6])
- Inflammatory bowel disease (Crohn’s disease and ulcerative colitis)
- Pregnancy
Less frequent and less definitive associations exist with other infections (eg, human immunodeficiency virus, tuberculosis, chlamydia, viral hepatitis), primary immunodeficiencies, and autoimmune conditions (eg, Behçet’s disease, relapsing polychondritis, rheumatoid arthritis, sarcoidosis, autoimmune thyroid disease, connective tissue disorders including systemic lupus erythematosus and dermatomyositis) [2,7]. Further studies are necessary to determine the strength of the relationships between Sweet syndrome and these diseases.
Malignancy-associated Sweet syndrome — Malignancy-associated Sweet syndrome accounts for a significant portion of cases of Sweet syndrome [8-10]. A 1993 review of several retrospective series found that 25 of 118 patients with Sweet syndrome (21 percent) had a hematologic or solid tumor malignancy [8]. In children, malignancy-associated Sweet syndrome appears to primarily occur in children over the age of three years [9]. Rare cases of malignancy-associated Sweet syndrome have been reported in infants [11,12].
Sweet syndrome may precede, follow, or appear concurrently with a malignancy [8,13-44]. In patients with previous histories of cancer, the development of Sweet syndrome may portend disease recurrence [2].
Sweet syndrome is more likely to occur in association with hematologic malignancies than solid tumor cancers. In a 1998 review of 79 patients with malignancies and Sweet syndrome, 69 (87 percent) had hematologic malignancies and 12 had solid tumors (15 percent), including two patients who had both acute myelogenous leukemia (AML) and prostate cancer [45]. AML is the malignancy most frequently associated with Sweet syndrome. In the review, AML accounted for 42 percent of the hematologic malignancies and myeloproliferative disorders comprised the second most common category of malignancy (22 percent) [45]. Among solid tumors, carcinomas of the genitourinary organs, breast, and gastrointestinal tract are most frequently linked with Sweet syndrome [8,46].
Drug-induced Sweet syndrome — Multiple drugs may contribute to Sweet syndrome [47-56]. A list of drugs that have been associated with Sweet syndrome is provided in a table. Granulocyte-colony stimulating factor (G-CSF) is the most widely reported contributory medication [50].
Sweet syndrome usually develops about two weeks after drug exposure in patients who lack a prior history of exposure to the inciting drug [57]. Recurrence of the syndrome usually develops after reexposure to the inciting drug [2].
EPIDEMIOLOGY — Although individuals between the ages of 30 and 60 most frequently develop Sweet syndrome, infants, children, and older adults may also be affected [7,9]. The mean age for reported pediatric cases is slightly over five years [9].
The sex distribution varies with the subtype of Sweet syndrome and patient age. In classical Sweet syndrome, 80 percent of patients are women, whereas the proportion of women is lower in other clinical variants [2]. In Sweet syndrome associated with drugs, solid tumors, and hematologic malignancies, the percentage of patients who are women is estimated to be 70, 60, and 50 percent [2]. The female predominance in Sweet syndrome does not appear to apply to children. In a systematic review that included 66 reports of children with Sweet syndrome, the disorder was twice as likely to occur in males among children under the age of three [9]. In children over the age of three, the sex distribution was equal.
There is no obvious racial predilection in Sweet syndrome.
PATHOGENESIS — The pathogenesis of Sweet syndrome is not well understood. Factors theorized to contribute to the development of this disorder include hypersensitivity reactions, cytokine dysregulation, and genetic susceptibility.
- Hypersensitivity reaction – An immune reaction to bacterial, viral, tumor, or other antigens could influence the development of Sweet syndrome through stimulating the production of cytokines that promote neutrophil activation and infiltration [58]. The positive response to systemic glucocorticoids and reports of improvement in Sweet syndrome following treatment of bacterial infections or malignancies offer some support to this theory [58-60].
- Cytokine dysregulation – Certain cytokines and chemokines may contribute to the initiation and propagation of the inflammatory response in Sweet syndrome. A role for granulocyte colony-stimulating factor (G-CSF) is compelling, since G-CSF increases circulating neutrophils and exogenous G-CSF is a common cause of drug-induced Sweet syndrome [50]. Moreover, higher serum levels of G-CSF have been detected in patients with active Sweet syndrome than in patients with inactive disease [61]. Increased production of G-CSF by tumor cells has also been proposed as a potential factor in malignancy-associated disease [2,62].
Other cytokines, including granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukins (eg, IL-1, IL-3, IL-6, and IL-8) are postulated to play roles in Sweet syndrome [63-66]. In a study of eight patients with Sweet syndrome, serum levels of Th1 cytokines, including IL-1α, IL-1β, IL-2, and interferon-gamma were elevated, whereas levels of IL-4, a Th2 cytokine remained normal [67]. - Genetic susceptibility – Abnormalities in chromosome 3q have been reported in association with Sweet syndrome in a few patients [31,68-70]. HLA-B54 has been linked to Sweet syndrome in Japanese patients [71,72].
Antibodies to neutrophilic cytoplasmic antigens have been detected in some patients with Sweet syndrome [70,73]. However, a pathogenic role for these antibodies has not been established. Further study is necessary to clarify the mechanisms that lead to the development of Sweet syndrome.
CLINICAL MANIFESTATIONS — In addition to the abrupt onset of the painful inflammatory papules, plaques, and nodules that characterize Sweet syndrome, additional clinical features may develop. An overview of the mucocutaneous and extracutaneous manifestations of Sweet syndrome is provided below. The differences in the clinical and laboratory features of the subtypes of Sweet syndrome are provided in a table.
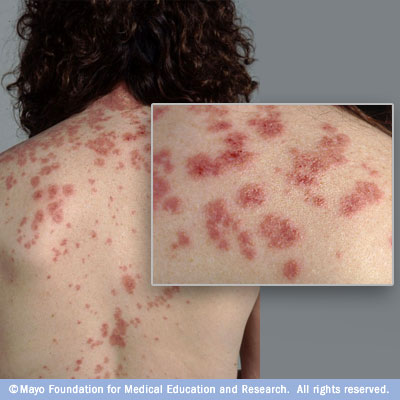
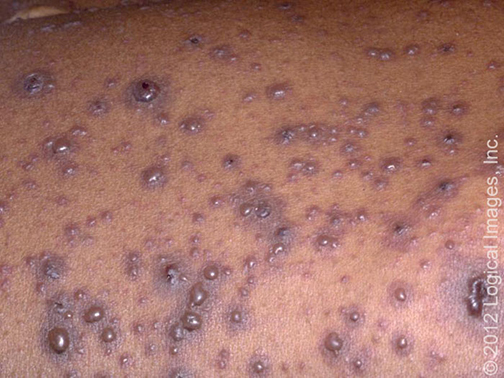
Cutaneous — The cutaneous lesions of Sweet syndrome typically present as tender, edematous, and inflamed papules, plaques, and nodules (picture 1A-B). Lesions are usually several millimeters to several centimeters in diameter and exhibit a brightly erythematous or violaceous color.
The inflammatory papules and plaques often exhibit a mamillated surface [74]. Significant superficial dermal edema leading to a pseudovesicular quality is common. Pustules may also be present. In some cases, the skin lesions develop a central yellowish hue resulting in a targetoid appearance.
The distribution of the cutaneous eruption is often asymmetrical. Overall, the head, neck, and upper extremities are favored sites for involvement. Skin lesions may also occur on the lower extremities in drug-induced or malignancy-associated disease, but are infrequently found on the lower extremities in classical Sweet syndrome.
Patients often describe the pain associated with cutaneous lesions in Sweet syndrome as tenderness or a burning sensation. Pruritus usually does not occur.
Less common presentations of Sweet syndrome include:
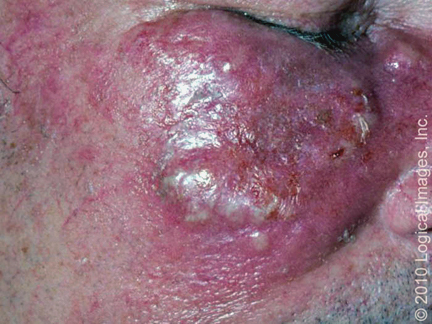
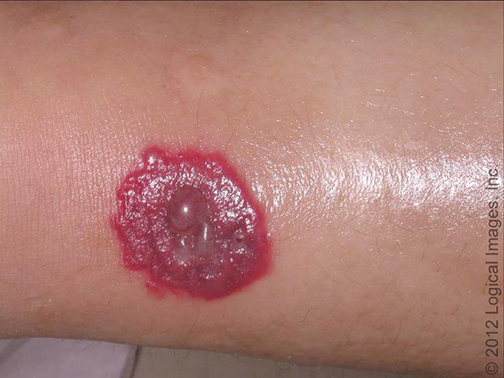
- Bullous Sweet syndrome – Bullous Sweet syndrome is an uncommon presentation of Sweet syndrome in which vesicles and flaccid bullae overly erythematous to violaceous plaques (picture 2A-B) [58]. Ulceration resembling pyoderma gangrenosum may occur in bullous Sweet syndrome; this manifestation most commonly occurs in the setting of hematologic malignancy [28,74].
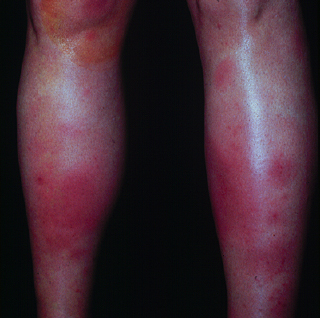
- Subcutaneous Sweet syndrome – In subcutaneous Sweet syndrome, the site of neutrophilic infiltration is in the subcutaneous fat, rather than the dermis (see 'Pathology findings' below). This results in the development of erythematous nodules with minimal superficial change. The nodules are usually 2 to 3 cm in diameter [75], and the extremities are common sites of involvement. Subcutaneous Sweet syndrome can closely resemble erythema nodosum, particularly when it involves the lower legs (picture 3) [76].
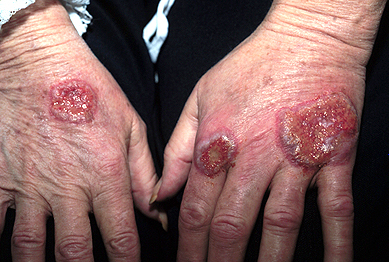
- Neutrophilic dermatosis of the dorsal hands – Neutrophilic dermatosis of the dorsal hands (also known as pustular vasculitis of the dorsal hands) is a localized disorder that may be on a continuum with Sweet syndrome [77]. Affected patients develop inflammatory and pustular plaques on the dorsal surfaces of the hands (picture 4). Neutrophilic dermatosis of the dorsal hands is discussed in greater detail separately. (See "Neutrophilic dermatoses", section on 'Neutrophilic dermatosis of the dorsal hands'.)
The phenomenon of pathergy (lesion development at sites of cutaneous injury) may occur in patients with Sweet syndrome [9]. Even minor trauma, such as that obtained with a needle stick, may result in the development of skin lesions.
Oral cavity — Oral involvement is uncommon in classical Sweet syndrome. However, approximately 12 percent of patients with Sweet syndrome related to hematologic malignancies develop oral ulcers as a disease manifestation, particularly on the buccal mucosa or tongue [2]. Additional reported oral findings include bullae, vesicles, gingival hyperplasia, necrotizing ulcerative periodontitis, nodules, papules, pustules, and tongue swelling [2,78].
Associated symptoms — The name “acute febrile neutrophilic dermatosis” reflects the frequent presence of fever >38°C in Sweet syndrome. Patients with drug-induced Sweet syndrome are almost always febrile, but fever may spare 10 to 20 percent of patients with classical or malignancy-associated disease [2]. Arthralgias, malaise, headache, and myalgias are additional symptoms that frequently occur in Sweet syndrome [6,74].
Extracutaneous disease — Neutrophilic infiltration of other organ systems, such as the eye, muscles, lung, bone, liver, spleen, heart, kidneys, central nervous system, and gastrointestinal system may occur in Sweet syndrome, resulting in signs or symptoms specific to the extracutaneous site of inflammation. Ocular inflammation is a common extracutaneous manifestation; ocular involvement occurs in 17 to 72 percent of patients with the classical variant [2]. Examples of ocular manifestations include conjunctivitis, episcleritis, scleritis, limbal nodules, peripheral ulcerative keratitis, iritis, glaucoma, dacryoadenitis, and choroiditis [79,80]. The musculoskeletal system is another frequent site of extracutaneous involvement, manifesting as arthralgias, arthritis, and myalgias.
Examples of uncommon extracutaneous manifestations of Sweet syndrome include [6,74]:
- Central nervous system – encephalitis, aseptic meningitis
- Cardiovascular system – myocarditis, aortitis and aortic stenosis, coronary artery occlusion
- Pulmonary system – neutrophilic alveolitis, pleural effusions, airway obstruction
- Liver – hepatitis, hepatomegaly
- Intestines – neutrophilic inflammation of intestines
- Spleen – splenomegaly
- Kidneys – mesangial glomerulonephritis, hematuria, proteinuria
- Bone – sterile osteomyelitis
The development of systemic inflammatory response syndrome (SIRS) in the setting of Sweet syndrome has also been reported [16,81-83].
Associated laboratory findings — Peripheral leukocytosis with neutrophilia is the most common laboratory abnormality in patients with Sweet syndrome. Neutrophilia is present in the vast majority of patients with classical Sweet syndrome, and in a significant proportion of patients with malignancy-associated and drug-induced disease. Nonspecific inflammatory markers such as the erythrocyte sedimentation rate and C-reactive protein level are also frequently elevated in patients with Sweet syndrome.
Anemia and platelet abnormalities are commonly seen in Sweet syndrome related to malignancy or drugs, but are infrequently detected in patients with classical Sweet syndrome. Abnormalities on the complete metabolic panel and urinalysis may indicate hepatic or renal involvement.
DIAGNOSIS — The diagnosis of Sweet syndrome is based upon the recognition of consistent clinical and laboratory findings as well as the exclusion of disorders that may present with similar clinical features. A rapid and dramatic response to systemic glucocorticoid therapy also supports the diagnosis.
Diagnostic criteria — A revised set of diagnostic criteria [84] based upon criteria outlined in 1986 [48] are widely used for the diagnosis of Sweet syndrome. Both major criteria and two of four minor criteria are required to establish a diagnosis of classical or malignancy-associated Sweet syndrome.
Major criteria:
- Abrupt onset of painful erythematous plaques or nodules
- Histopathologic evidence of a dense neutrophilic infiltrate without evidence of leukocytoclastic vasculitis
Minor criteria:
- Pyrexia >38°C
- Association with underlying hematologic or visceral malignancy, inflammatory disease or pregnancy, OR preceded by upper respiratory infection, gastrointestinal infection, or vaccination
- Excellent response to treatment with systemic glucocorticoids or potassium iodide
- Abnormal laboratory values at presentation (three of four of the following: erythrocyte sedimentation rate >20 mm/hr, positive C-reactive protein, >8,000 leukocytes, >70 percent neutrophils)
A separate set of criteria have been proposed for drug-induced Sweet syndrome [47].
Clinical assessment — The clinical assessment of the patient offers valuable clues for diagnosis. The history may reveal a rapid onset of skin lesions and the presence of associated symptoms (eg, painful skin lesions, fever, malaise), findings that are compatible with Sweet syndrome. In addition, the knowledge that a patient has a condition known to occur in association with Sweet syndrome (eg, recent infection, malignancy, pregnancy, or inflammatory bowel disease) raises clinical suspicion for the diagnosis. (See 'Clinical manifestations' above and 'Classification' above.)
A complete physical examination that includes surveillance of the entire skin surface should be performed. This aids in determining whether the morphology and distribution of skin lesions is consistent with Sweet syndrome and allows for an assessment of the extent of cutaneous disease as well as an evaluation for signs of extracutaneous involvement.
Laboratory studies — In patients in whom the clinical findings suggest the possibility of Sweet syndrome, a skin biopsy and select laboratory tests are indicated.
Biopsy — The detection of consistent histological findings is a major diagnostic criterion for Sweet syndrome (see 'Diagnostic criteria' above). Thus, a skin biopsy should be performed whenever feasible.
Procedure — In patients with inflammatory papules or plaques, a 4 mm punch biopsy is usually sufficient for obtaining a tissue specimen for histological examination. Because infection may closely resemble Sweet syndrome, microbial pathology stains should be performed. In addition, we obtain a second 4 mm punch biopsy for bacterial, fungal, and mycobacterial cultures. If pustules are present, a swab specimen can also be sent for culture.
In patients with nodules suggestive of subcutaneous Sweet syndrome (a variant in which the pathologic process is primarily located in the subcutaneous fat), an excisional biopsy is preferred because it provides a more generous sample of the subcutaneous fat, which may facilitate the interpretation of the histological findings. As above, a portion of the tissue specimen should be sent for microbial cultures. (See 'Pathology findings' below and "Skin biopsy techniques" and "Panniculitis: Recognition and diagnosis", section on 'Biopsy'.)
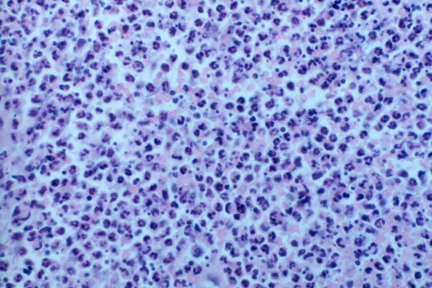
Pathology findings — The characteristic histologic features of Sweet syndrome include [85]:
- Prominent edema in the superficial dermis
- Dense infiltrate of neutrophils in the upper and mid-dermis with sparing of the epidermis (picture 5)
- Leukocytoclasis
- Endothelial swelling
- Absence of vasculitis (see below)
A few eosinophils may also be present. Older lesions may exhibit small numbers of lymphocytes or macrophages [85].
Although the absence of vasculitis is considered a characteristic feature of Sweet syndrome, the identification of vasculitis in a biopsy specimen does not rule out the diagnosis. In a retrospective study of skin biopsies from 21 patients with Sweet syndrome, vasculitis was identified in specimens from 6 patients (29 percent) [86]. The vasculitis seen in Sweet syndrome may be a secondary phenomenon related to the release of noxious products from neutrophils [86].
Pathological variants of Sweet syndrome include subcutaneous Sweet syndrome and histiocytoid Sweet syndrome [87-91]. Subcutaneous Sweet syndrome is characterized by a dense neutrophilic infiltrate in the subcutaneous tissue. Although lobular inflammation is usually most prominent, both septa and lobules may be involved [75]. In histiocytoid Sweet syndrome, the dermal inflammatory infiltrate is primarily composed of histiocyte-like immature myeloid cells [92].
The histologic differential diagnosis of Sweet syndrome is provided in a table.
Serologic and other tests — Laboratory evaluation is useful for identifying findings consistent with Sweet syndrome (eg, leukocytosis) and signs of associated diseases or extracutaneous involvement. Our routine laboratory work-up for patients with suspected Sweet syndrome includes the following:
- Complete blood count with platelets and differential
- Complete metabolic panel
- Erythrocyte sedimentation rate or C-reactive protein
- Urinalysis
- Pregnancy test in women of childbearing age
Additional laboratory studies are ordered based upon suspicion for specific sites of extracutaneous disease and associated underlying disorders. For example, patients with pulmonary symptoms, pleuritis, or hypoxia should have a chest radiograph to look for signs of pulmonary involvement. Examples of additional investigations for an underlying disorder include searches for evidence of recent streptococcal infection or an occult malignancy.
Evaluation for malignancy — Malignancy testing should only be considered in the setting of reasonable clinical suspicion for an underlying malignancy (eg, constitutional symptoms such as weight loss) and the absence of another explanation for a Sweet syndrome diagnosis (ie, pregnancy, drug exposure, recent infection, inflammatory bowel disease, rheumatoid arthritis, etc.). The screening for malignancy should begin with age-appropriate cancer screening guidelines. Some experts have proposed a work-up based upon the most common malignancies reported in association with Sweet syndrome [8]. This includes a physical examination that incorporates thyroid and lymph node examinations, as well as breast, cervical, uterine, and ovarian cancer screening in women and prostate and testicular examinations in men. Additional tests considered by these authors include a carcinoembryonic antigen level, colon cancer screening, and a chest radiograph. Other authors have suggested the use of chest, abdomen, and pelvis CT imaging or PET-CT imaging to evaluate for malignancy in patients with paraneoplastic conditions and reasonable suspicion for a malignancy of unknown source; this is similar to the literature for malignancy work-up of dermatomyositis, which is largely driven by expert opinion [93-95].
DIFFERENTIAL DIAGNOSIS — Infection is one of the most important disorders to consider in the patient with clinical and/or histological findings suggestive of Sweet syndrome. As in Sweet syndrome, fever, leukocytosis, and cutaneous lesions characterized by neutrophil-dense infiltrates may occur in bacterial sepsis. In addition, the lesions of Sweet syndrome can resemble local bacterial, fungal, or atypical mycobacterial infections.
The clinical differential diagnosis of Sweet syndrome is dependent upon lesion morphology. Data obtained from the clinical history, physical examination, pathology findings, and microbial studies are useful for distinguishing between Sweet syndrome and other conditions.
- Erythematous, edematous plaques:
- Cutaneous infection (bacterial, fungal, and mycobacterial)
- Urticaria and urticarial vasculitis
- Other neutrophilic dermatoses (eg, pyoderma gangrenosum, neutrophilic eccrine hidradenitis, Behçet's disease, cutaneous metastatic Crohn’s disease)
- Drug eruptions
- Halogenoderma (eg, bromoderma, iododerma)
- Nodules (including subcutaneous Sweet syndrome):
- Cutaneous infection (eg, deep fungal infection, Majocchi’s granuloma, atypical mycobacterial infection)
- Malignancy (eg, lymphoma cutis, leukemia cutis, metastatic carcinoma)
- Subcutaneous sarcoidosis
- Vasculitis (particularly medium-vessel vasculitis such as cutaneous polyarteritis nodosa)
- Erythema nodosum
- Bullous lesions:
- Bullous pyoderma gangrenosum
- Bullous leukocytoclastic vasculitis
- Autoimmune bullous diseases (eg, bullous pemphigoid, bullous systemic lupus erythematosus, inflammatory epidermolysis bullosa acquisita, linear IgA bullous dermatosis)
- Infection with bullous and hemorrhagic or necrotic changes (eg, bullous cellulitis, vessel invasive infections such as aspergillosis, ecthyma gangrenosum).
The histopathologic differential diagnosis of Sweet syndrome is reviewed in a table.
SUMMARY AND RECOMMENDATIONS
- Sweet syndrome (acute febrile neutrophilic dermatosis) is an inflammatory disorder characterized by the presence of inflammatory papules, plaques, or nodules on the skin, systemic symptoms, and neutrophilic infiltration of the skin. Sweet syndrome is often divided into three categories based on etiology: classical Sweet syndrome, malignancy-associated Sweet syndrome, and drug-induced Sweet syndrome. (See 'Classification' above.)
- Classical Sweet syndrome includes cases of Sweet syndrome that are not associated with malignancy or drug exposure. The most common causes of malignancy-associated Sweet syndrome and drug-induced Sweet syndrome are acute myelogenous leukemia and granulocyte-colony stimulating factor (G-CSF), respectively. (See 'Classification' above.)
- The cutaneous lesions of Sweet syndrome are typically painful, erythematous to violaceous papules and plaques. Pseudovesiculation and pustule formation are often present. Bullous Sweet syndrome and subcutaneous Sweet syndrome are less common manifestations. (See 'Cutaneous' above.)
- Fever, malaise, joint pain, and muscle pain often accompany the cutaneous lesions. Involvement of internal organs may also occur. Leukocytosis and elevations in the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level are common laboratory findings. (See 'Associated symptoms' above and 'Extracutaneous disease' above and 'Associated laboratory findings' above.)
- A diagnosis of Sweet syndrome is made based upon both clinical and laboratory findings. Biopsies characteristically demonstrate a dense neutrophilic infiltrate in the dermis without vasculitis. (See 'Diagnosis' above.)
A brightly erythematous plaque with a pustular component is visible on this patient with Sweet's syndrome. Reproduced with permission from: www.visualdx.com. Copyright Logical Images, Inc.
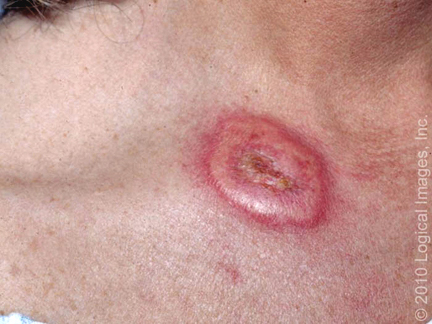
A brightly erythematous plaque with a pustular component is present on the face of this patient with Sweet's syndrome. Reproduced with permission from: www.visualdx.com. Copyright Logical Images, Inc.
Vesiculation is present in this inflammatory plaque of Sweet's syndrome. Reproduced with permission from: www.visualdx.com. Copyright Logical Images, Inc.
Vesicles and inflammatory papules and plaques are present in this patient with Sweet's syndrome. Reproduced with permission from: www.visualdx.com. Copyright Logical Images, Inc.
Erythema nodosum-like lesions of the lower extremities in a patient with Sweet's syndrome. Courtesy of Samuel Moschella, MD.
Painful plaques that have progressed to ulceration. These developed in a 60-year-old female with seropositive rheumatoid arthritis. The histologic appearance was epithelial hyperplasia with intraepithelial sterile abscesses and dense dermal infiltrate of neutrophils with an absence of leucocytoclastic vasculitis. Courtesy of Samuel L Moschella, MD.
Dense neutrophilic infiltrate in Sweet's syndrome.
REFERENCES
- SWEET RD. AN ACUTE FEBRILE NEUTROPHILIC DERMATOSIS. Br J Dermatol 1964; 76:349.
- Cohen PR. Sweet's syndrome--a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis 2007; 2:34.
- Lallas A, Tzellos TG, Papageorgiou M, Mandekou-Lefaki I. Sweet's syndrome associated with upper respiratory tract streptococcal infection: "wait-and-see" strategy or anecdotal use of corticosteroids? Hippokratia 2011; 15:283.
- Ytting H, Vind I, Bang D, Munkholm P. Sweet's syndrome--an extraintestinal manifestation in inflammatory bowel disease. Digestion 2005; 72:195.
- Satra K, Zalka A, Cohen PR, Grossman ME. Sweet's syndrome and pregnancy. J Am Acad Dermatol 1994; 30:297.
- Cohen PR, Hongsmann H, Kurzrock R. Acute febrile neutrophilic dermatosis (Sweet syndrome). In: Fitzpatrick's Dermatology in General Medicine, 8th ed, Goldsmith LA, Katz SI, Gilchrest BA, et al.. (Eds), McGraw Hill, 2012. Vol 1, p.362.
- Gray PE, Bock V, Ziegler DS, Wargon O. Neonatal Sweet syndrome: a potential marker of serious systemic illness. Pediatrics 2012; 129:e1353.
- Cohen PR, Kurzrock R. Sweet's syndrome and cancer. Clin Dermatol 1993; 11:149.
- Halpern J, Salim A. Pediatric sweet syndrome: case report and literature review. Pediatr Dermatol 2009; 26:452.
- Bourke JF, Keohane S, Long CC, et al. Sweet's syndrome and malignancy in the U.K. Br J Dermatol 1997; 137:609.
- Uihlein LC, Brandling-Bennett HA, Lio PA, Liang MG. Sweet syndrome in children. Pediatr Dermatol 2012; 29:38.
- Krilov LR, Jacobson M, Shende A. Acute febrile neutrophilic dermatosis (Sweet's syndrome) presenting as facial cellulitis in a child with juvenile chronic myelogenous leukemia. Pediatr Infect Dis J 1987; 6:77.
- Miranda CV, Filgueiras Fde M, Obadia DL, et al. Sweet's Syndrome associated with Hodgkin's disease: case report. An Bras Dermatol 2011; 86:1016.
- Wawrzycki B, Chodorowska G, Pietrzak A, et al. Recurrent skin eruption in patient with chronic lymphocytic leukemia and lymphocytic infiltrates of the dermis resembling Sweet's syndrome. G Ital Dermatol Venereol 2011; 146:487.
- Ravaglia C, Poletti G, Gurioli C, et al. Sweet's syndrome associated with myelogenous leukemia and pulmonary involvement. Monaldi Arch Chest Dis 2011; 75:149.
- Shugarman IL, Schmit JM, Sbicca JA, Wirk B. Easily missed extracutaneous manifestation of malignancy-associated Sweet's syndrome: systemic inflammatory response syndrome. J Clin Oncol 2011; 29:e702.
- Kato T, Kawana S, Takezaki S, et al. Case of Sweet's syndrome with extensive necrosis and ulcers accompanied by myelodysplastic syndrome. J Nihon Med Sch 2008; 75:162.
- Glendenning J, Khoo V. Sweet's syndrome in prostate cancer. Prostate Cancer Prostatic Dis 2008; 11:397.
- Awan F, Hamadani M, Devine S. Paraneoplastic Sweet's syndrome and the pathergy phenomenon. Ann Hematol 2007; 86:613.
- Culp L, Crowder S, Hatch S. A rare association of Sweet's syndrome with cervical cancer. Gynecol Oncol 2004; 95:396.
- Dereure O, Ebrard-Charra S, Guillon F, et al. Sweet's syndrome associated with pheochromocytoma. Dermatology 2004; 208:175.
- Liu D, Seiter K, Mathews T, et al. Sweet's syndrome with CML cell infiltration of the skin in a patient with chronic-phase CML while taking Imatinib Mesylate. Leuk Res 2004; 28 Suppl 1:S61.
- Gille J, Spieth K, Kaufmann R. Sweet's syndrome as initial presentation of diffuse large B-cell lymphoma. J Am Acad Dermatol 2002; 46:S11.
- Zappasodi P, Corso A, del Forno C. Sweet's syndrome and myelodysplasia: two entities with a common pathogenetic mechanism? A case report. Haematologica 2000; 85:868.
- Suvajdzić N, Dimcić Z, Cvijetić O, Colović M. Sweet's syndrome associated with Hodgkin's disease. Haematologia (Budap) 1998; 29:157.
- Aubin F, Dufour MP, Angonin R, et al. Sweet's syndrome associated with cutaneous T cell lymphoma. Eur J Dermatol 1998; 8:178.
- Cho KH, Han KH, Kim SW, et al. Neutrophilic dermatoses associated with myeloid malignancy. Clin Exp Dermatol 1997; 22:269.
- Lear JT, Byrne JP. Bullous pyoderma gangrenosum, Sweet's syndrome and malignancy. Br J Dermatol 1997; 136:296.
- Deguchi M, Tsunoda T, Yuda F, Tagami H. Sweet's syndrome in acute myelogenous leukemia showing dermal infiltration of leukemic cells. Dermatology 1997; 194:182.
- Woodrow SL, Munn SE, Basarab T, Russel Jones R. Sweet's syndrome in association with non-Hodgkin's lymphoma. Clin Exp Dermatol 1996; 21:357.
- Colović MD, Janković GM, Novak AZ, et al. Sweet's syndrome associated with paracentric inversion of chromosome 3q in a patient with multiple myeloma. Eur J Haematol 1996; 57:188.
- Heer-Sonderhoff AH, Arning M, Wehmeier A, et al. Neutrophilic dermal infiltrates in granulocytopenic patients with acute leukemia. Ann Hematol 1995; 71:257.
- Shirono K, Kiyofuji C, Tsuda H. Sweet's syndrome in a patient with acute promyelocytic leukemia during treatment with all-trans retinoic acid. Int J Hematol 1995; 62:183.
- Yamamoto T, Furuse Y, Nishioka K. Sweet's syndrome with small cell carcinoma of the lung. J Dermatol 1994; 21:125.
- van Kamp H, van den Berg E, Timens W, et al. Sweet's syndrome in myeloid malignancy: a report of two cases. Br J Haematol 1994; 86:415.
- Inanç SE, Altum M, Onat H, Erseven G. Sweet's syndrome and Hodgkin's disease. Acta Oncol 1994; 33:574.
- Barnadas MA, Sitjàs D, Brunet S, et al. Acute febrile neutrophilic dermatosis (Sweet's syndrome) associated with prostate adenocarcinoma and a myelodysplastic syndrome. Int J Dermatol 1992; 31:647.
- Kramers C, Raemaekers JM, van Baar HM, et al. Sweet's syndrome as the presenting symptom of hairy cell leukemia with concomitant infection by Mycobacterium kansasii. Ann Hematol 1992; 65:55.
- Torralbo A, Herrero JA, del-Rio E, et al. Sweet's syndrome associated with multiple myeloma. Int J Dermatol 1992; 31:297.
- Uchida H, Ikari Y, Hashizume S, et al. A case of Sweet's syndrome with early gastric cancer. Dermatologica 1990; 181:224.
- Fischer G, Commens C, Bradstock K. Sweet's syndrome in hairy cell leukemia. J Am Acad Dermatol 1989; 21:573.
- Tuncer AM. Acute lymphoblastic leukemia and Sweet's syndrome. Acta Haematol 1988; 80:224.
- Visani G, Patrizi AL, Ricci P, et al. Sweet's syndrome: association with accelerated phase of chronic myeloid leukemia. Acta Haematol 1988; 79:207.
- Nguyen KQ, Hurst CG, Pierson DL, Rodman OG. Sweet's syndrome and ovarian carcinoma. Cutis 1983; 32:152.
- Cohen PR, Talpaz M, Kurzrock R. Malignancy-associated Sweet's syndrome: review of the world literature. J Clin Oncol 1988; 6:1887.
- Cohen PR, Holder WR, Tucker SB, et al. Sweet syndrome in patients with solid tumors. Cancer 1993; 72:2723.
- Walker DC, Cohen PR. Trimethoprim-sulfamethoxazole-associated acute febrile neutrophilic dermatosis: case report and review of drug-induced Sweet's syndrome. J Am Acad Dermatol 1996; 34:918.
- Su WP, Liu HN. Diagnostic criteria for Sweet's syndrome. Cutis 1986; 37:167.
- Magro CM, De Moraes E, Burns F. Sweet's syndrome in the setting of CD34-positive acute myelogenous leukemia treated with granulocyte colony stimulating factor: evidence for a clonal neutrophilic dermatosis. J Cutan Pathol 2001; 28:90.
- White JM, Mufti GJ, Salisbury JR, du Vivier AW. Cutaneous manifestations of granulocyte colony-stimulating factor. Clin Exp Dermatol 2006; 31:206.
- Treton X, Joly F, Alves A, et al. Azathioprine-induced Sweet's syndrome in Crohn's disease. Inflamm Bowel Dis 2008; 14:1757.
- Juanola X, Nolla JM, Servitje O, Valverde J. Hydralazine induced lupus and Sweet's syndrome. J Rheumatol 1991; 18:948.
- Thibault MJ, Billick RC, Srolovitz H. Minocycline-induced Sweet's syndrome. J Am Acad Dermatol 1992; 27:801.
- Mensing H, Kowalzick L. Acute febrile neutrophilic dermatosis (Sweet's syndrome) caused by minocycline. Dermatologica 1991; 182:43.
- Park CJ, Bae YD, Choi JY, et al. Sweet's syndrome during the treatment of acute promyelocytic leukemia with all-trans retinoic acid. Korean J Intern Med 2001; 16:218.
- Arun B, Berberian B, Azumi N, et al. Sweet's syndrome during treatment with all-trans retinoic acid in a patient with acute promyelocytic leukemia. Leuk Lymphoma 1998; 31:613.
- Kim MJ, Choe YH. EPONYM. Sweet syndrome. Eur J Pediatr 2010; 169:1439.
- Voelter-Mahlknecht S, Bauer J, Metzler G, et al. Bullous variant of Sweet's syndrome. Int J Dermatol 2005; 44:946.
- Escallier F, Gaudard S, Courtois JM, et al. [Sweet's syndrome and Yersinia enterocolitica infection]. Ann Dermatol Venereol 1990; 117:858.
- O'Connor Reina C, Garcia Iriarte MT, Rodriguez Diaz A, et al. Tonsil cancer and Sweet's syndrome. Otolaryngol Head Neck Surg 1998; 119:709.
- Kawakami T, Ohashi S, Kawa Y, et al. Elevated serum granulocyte colony-stimulating factor levels in patients with active phase of sweet syndrome and patients with active behcet disease: implication in neutrophil apoptosis dysfunction. Arch Dermatol 2004; 140:570.
- Shinojima Y, Toma Y, Terui T. Sweet syndrome associated with intrahepatic cholangiocarcinoma producing granulocyte colony-stimulating factor. Br J Dermatol 2006; 155:1103.
- Tanaka N, Fujioka A, Tajima S, et al. Elafin is induced in epidermis in skin disorders with dermal neutrophilic infiltration: interleukin-1 beta and tumour necrosis factor-alpha stimulate its secretion in vitro. Br J Dermatol 2000; 143:728.
- Reuss-Borst MA, Müller CA, Waller HD. The possible role of G-CSF in the pathogenesis of Sweet's syndrome. Leuk Lymphoma 1994; 15:261.
- Loraas A, Waage A, Lamvik J. Cytokine response pattern in Sweet's syndrome associated with myelodysplasia. Br J Haematol 1994; 87:669.
- Reuss-Borst MA, Pawelec G, Saal JG, et al. Sweet's syndrome associated with myelodysplasia: possible role of cytokines in the pathogenesis of the disease. Br J Haematol 1993; 84:356.
- Giasuddin AS, El-Orfi AH, Ziu MM, El-Barnawi NY. Sweet's syndrome: is the pathogenesis mediated by helper T cell type 1 cytokines? J Am Acad Dermatol 1998; 39:940.
- Mijovic A, Novak A, Medenica L. Sweet's syndrome associated with inversion of chromosome 3q in a patient with refractory anemia. Eur J Haematol 1992; 49:156.
- Billström R, Heim S, Kristoffersson U, et al. Structural chromosomal abnormalities of 3q in myelodysplastic syndrome/acute myeloid leukaemia with Sweet's syndrome. Eur J Haematol 1990; 45:150.
- Kemmett D, Harrison DJ, Hunter JA. Antibodies to neutrophil cytoplasmic antigens: serologic marker for Sweet's syndrome. J Am Acad Dermatol 1991; 24:967.
- Takahama H, Kanbe T. Neutrophilic dermatosis of the dorsal hands: a case showing HLA B54, the marker of Sweet's syndrome. Int J Dermatol 2010; 49:1079.
- Mizoguchi M, Matsuki K, Mochizuki M, et al. Human leukocyte antigen in Sweet's syndrome and its relationship to Behçet's disease. Arch Dermatol 1988; 124:1069.
- von den Driesch P, Weber MF. Are antibodies to neutrophilic cytoplasmic antigens (ANCA) a serologic marker for Sweet's syndrome? J Am Acad Dermatol 1993; 29:666.
- Moschella SL, Davis M. Neutrophilic dermatoses. In: Dermatology, Bolognia JL, Jorizzo JL, Rapini RP. (Eds), Elsevier, 2008. Vol 1, p.379.
- Guhl G, García-Díez A. Subcutaneous sweet syndrome. Dermatol Clin 2008; 26:541.
- Cohen PR. Subcutaneous Sweet's syndrome: a variant of acute febrile neutrophilic dermatosis that is included in the histopathologic differential diagnosis of neutrophilic panniculitis. J Am Acad Dermatol 2005; 52:927.
- Walling HW, Snipes CJ, Gerami P, Piette WW. The relationship between neutrophilic dermatosis of the dorsal hands and sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol 2006; 142:57.
- Bamelis M, Boyden B, Sente F, Madoe V. Sweet's syndrome and acute myelogenous leukemia in a patient who presented with a sudden massive swelling of the tongue. Dermatology 1995; 190:335.
- Gottlieb CC, Mishra A, Belliveau D, et al. Ocular involvement in acute febrile neutrophilic dermatosis (Sweet syndrome): new cases and review of the literature. Surv Ophthalmol 2008; 53:219.
- Medenblik-Frysch S, von den Driesch P, Jonas JB, Meythaler FH. Ocular complications in Sweet's syndrome. Am J Ophthalmol 1992; 114:230.
- Vanourny J, Swick BL. Sweet syndrome with systemic inflammatory response syndrome. Arch Dermatol 2012; 148:969.
- Sawicki J, Morton RA, Ellis AK. Sweet syndrome with associated systemic inflammatory response syndrome: an ultimately fatal case. Ann Allergy Asthma Immunol 2010; 105:321.
- Otheo E, Ros P, Vázquez JL, et al. Systemic inflammatory response syndrome associated with Sweet's syndrome. Pediatr Crit Care Med 2002; 3:190.
- von den Driesch P. Sweet's syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol 1994; 31:535.
- Weedon D. The vasculopathic reaction pattern. In: Weedon's Skin Pathology, 3rd ed, Elsevier Limited, 2010. p.195.
- Malone JC, Slone SP, Wills-Frank LA, et al. Vascular inflammation (vasculitis) in sweet syndrome: a clinicopathologic study of 28 biopsy specimens from 21 patients. Arch Dermatol 2002; 138:345.
- Lin J, Zhang Q, Chen M. Subcutaneous histiocytoid Sweet's syndrome in a patient associated with myelodysplastic syndrome-refractory anemia. J Dermatol 2012; 39:99.
- Kaiser R, Connolly K, Linker C, et al. Stem cell transplant for myelodysplastic syndrome-associated histiocytoid Sweet's syndrome in a patient with arthritis and myalgias. Arthritis Rheum 2008; 59:1832.
- Spencer B, Nanavati A, Greene J, Butler DF. Dapsone-responsive histiocytoid Sweet's syndrome associated with Crohn's disease. J Am Acad Dermatol 2008; 59:S58.
- Wu AJ, Rodgers T, Fullen DR. Drug-associated histiocytoid Sweet's syndrome: a true neutrophilic maturation arrest variant. J Cutan Pathol 2008; 35:220.
- Chow S, Pasternak S, Green P, et al. Histiocytoid neutrophilic dermatoses and panniculitides: variations on a theme. Am J Dermatopathol 2007; 29:334.
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol 2005; 141:834.
- Wasson S, Govindarajan G, Folzenlogen D. Concurrent occurrence of Sweet's syndrome and erythema nodosum: an overlap in the spectrum of reactive dermatoses. Clin Rheumatol 2006; 25:268.
- Titulaer MJ, Soffietti R, Dalmau J, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS task force. Eur J Neurol 2011; 18:19.
- Gainon J, Bart PA, Waeber G. [Can we predict the risk of malignancy associated with dermatomyositis?]. Praxis (Bern 1994) 2003; 92:1734.





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 